Investors in the seed round include SOSV, Zero Carbon Capital, Marinya Capital and angel Sake Bosch, with an equity-free grant also received from the UK Research and Innovation Council.
Funding will be used to take Multus Biotechnology’s first product to market by the end of 2021, before scaling production in 2022 to support the regulatory approval and commercialisation of cultivated meat, co-founder Cai Linton explained.
“Our plan is that by 2027, we want to see cultivated meat in the supermarkets competing with conventional meat – that’s the guiding light we’re aiming for.”
‘Tackling the growth media challenge’
Multus was founded by CEO Linton, CTO Kevin Pan, and COO Reka Tron in early 2019. The trio met through Imperial College’s Synthetic Biology Society, where they all shared ‘excitement’ for the potential of cultivated meat, Linton recalled. “We really wanted to tackle the key technical challenges that are holding this space back.”
In a more global sense, Multus is wanting to play its part in fixing ‘a broken food system’. “Industrial meat production is damaging the planet,” the CEO told FoodNavigator. “It is broken in so many ways, from animal welfare, to the climate, land use, and water use… We saw our background in bioscience and engineering as a way to solve that problem through cultivated meat.”

Upon digging deeper into the space, the co-founders pinpointed a key barrier in scaling up production.
“Growth media kept coming up again and again as the element really driving the cost of production to heights that were unsustainable for any kind of scale-up, and then to commercialisation. So we decided that this was the challenge we wanted to tackle.”
Alt foetal bovine serum
Multus’ solution is a ‘true replacement’ to foetal bovine serum (FBS) – a blood serum used in biomedical research, as well as in biopharmaceutical and therapeutical production.
FBS is usually extracted from bovines during the slaughtering process. From a scientific perspective, FBS has a great number of benefits, including its ability to encourage significant cell growth ‘to a high degree’.
“The key issues,” according to Linton, “are that it’s animal-derived, there is a lot of variability in its cost and performance, and in terms of scaling up to the volumes [required by] the cultivated meat industry, its potential is limited.”
Multus’ alternative to FBS, on the other hand, is completely animal-free, and designed specifically for the cultivated meat industry.
To tackle the issues surrounding growth media performance, cost, and scale, Multus is homing in on formulation and plant-based ingredients.
“One of our core technologies is that potent engineering piece,” the CEO explained. “Like many, we use growth factors as a serum replacement, but then on the nutrient side, we are looking at different extracts and hydrolysates to offset some of the individually purified ingredients that we use today.”
Growth factors are vital for signalling different processes that support cell growth, he elaborated. Yet current growth factors used by the industry tend to degrade quickly. This means that the whole growth media needs to be changed every two to three days. “This not only makes for a very laborious process, but also a very expensive process.”
To combat this issue, Multus is engineering proteins to maintain their core active function, extending different biophysical properties such as stability, solubility, and bioreactivity. This, Linton continued, makes them suitable for very large-scale production, and ‘really drives down their cost’ in terms of performance.
The start-up hasn’t yet run the numbers on exactly how much longer its growth media can be used, compared to others on the market, but the CEO suggested early data looks promising.
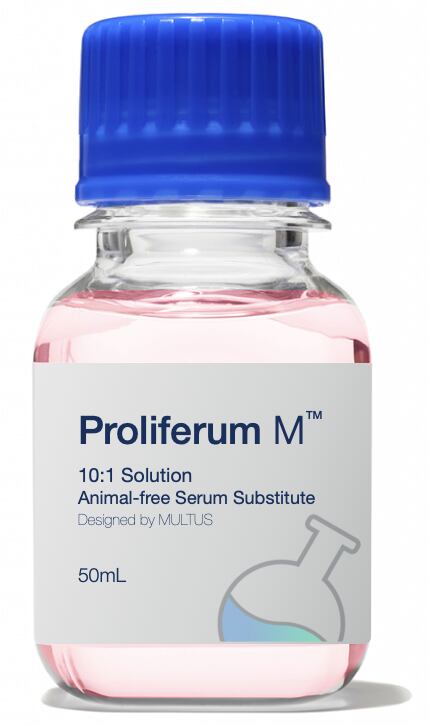
Commercialisation strategy
Multus’ first product, Proliferum M, is designed for mammalian cell proliferation, as used in cultured beef and pork products. “We are testing early formulation products at the moment,” we were told, “and seeking feedback and early validation before we roll out more broadly towards the end of this year”.
While the start-up is currently focused on cultivated beef and pork opportunities, it has plans to diversify its portfolio. “Within our pipeline, we [expect to] create a whole catalogue of standardised growth media for all cell types across cultivated meat.”
Concerning regulation, Multus is not faced with the same challenges as manufacturers – who are the ones submitting applications for commercial approval. However, the CEO said the company will be ‘supporting clients through that process’.
“Any persistent materials in that final product will be scrutinised,” he explained. “And in the US, growth media ingredients are likely to be seen as ingredients themselves.”
Multus is first eyeing the European market to test and validate its first product. But ‘very quickly’, Linton expects Multus to become a ‘global operation’, with key markets including Europe, North America, Singapore and China.





