Mike Robach, also VP, corporate food safety, quality & regulatory for Cargill, Inc. USA, spoke to FoodQualityNews at the Global Food Safety Conference in Houston, Texas. This is part two of the interview – read part one here.
He said the accredited third party certification programme system is full of checks and balances.
“We work with the International Accreditation Forum who has accreditation bureaus around the world. We have an integrity program within GFSI so we work with our certification process owners and audit them to assure they are doing the things they need to do to maintain the integrity of their programmes.
“They in turn then work with certification bodies and authorise them to issue certificates of their programs and in doing so, they also go in and assess what kind of job the certification bodies are doing.”
Auditor is where the rubber meets the road
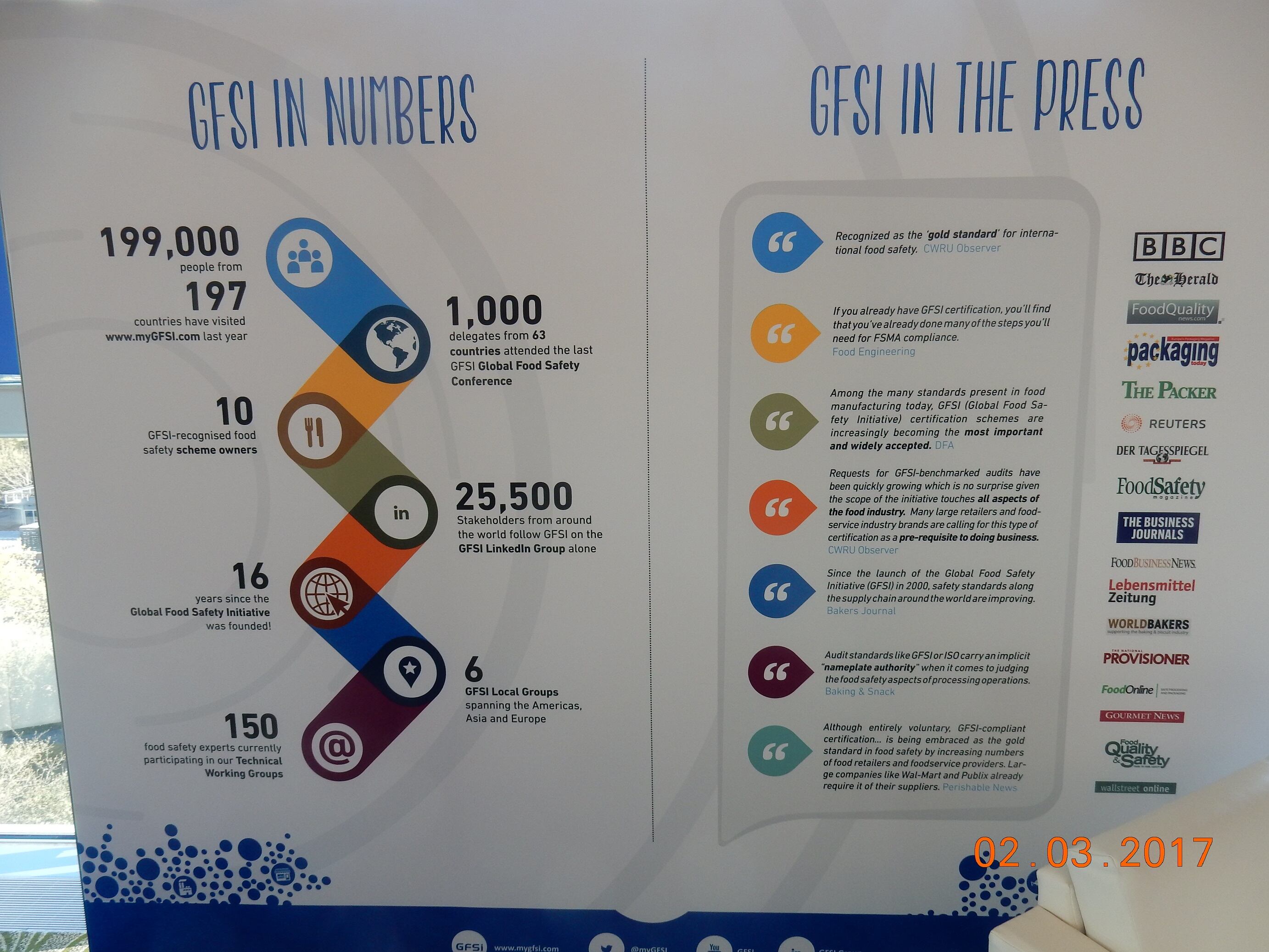
One of the important changes in the benchmarking requirements was around auditor competence.
“That was something that was in the past left to the individual process owner and GFSI felt we had to have a little more rigour around the knowledge and skills and performance of the auditor,” said Robach.
“The auditor is where the rubber meets the road and in talking with other stakeholders having this programme in place was essential to build trust in the certification process.”
Robach said it is not taking away the authority of national governments and regulatory agencies.
“Our premise is that it is the responsibility of the industry to produce save food and so GFSI allows us to have audits done and certificates issued that give us confidence that the systems are working as they are designed to work. At the same time, governments can use this certification as an element of their risk assessment,” he said.
“When they are looking at ‘Ok, I have so many inspectors and I have so many plants, where can I best spend my time to protect public health’. One of the things we are hoping they can do is take into account that if you have a certificate that you are operating at a level that is consistent with safe food.
“So governments can hopefully use that to make a decision on how they allocate resources. We look at what we do as complementary, they have regulatory oversight responsibility, that also builds consumer trust.
“We feel the work we are doing should be taken into consideration by the governments as they look at how they implement their regulatory programmes.”
System or implementation failure
Everybody has to understand a certificate does not ensure safe food, said Robach.
“We have two elements that we have to look at, one is around the systems that are in place so when you have an incident you have to determine was there a system failure or did we have an implementation failure. Do we have a system that is not capable or did something go wrong in the facility,” he said.
“It is important that people go through that root cause analysis to understand what happens. So there is a responsibility of the certification body to look at the circumstances and work with the company on the circumstances behind what happened.

“I know having been involved in situations within Cargill when we have an incident we do a root cause analysis, you figure out what went wrong and you correct it.
“The beauty of the GFSI forum is that we have an opportunity to share that and we do case studies on failures so people can learn from one another.”
Cargill: Category impact and implementing approaches
Speaking about Cargill, Robach said it looks at food safety as being pre-competitive.
“When one of our fellow companies fall down and make a mistake and there is a recall and illnesses it is not just that company that is impacted, it is the entire category,” he said.
“If you have a ground beef recall for example, it impacts the whole ground beef category regardless of producer. It is not about a single manufacturer or a single brand it is about the category. So we need to help each other to do better.
“When I talk about it being non-competitive that is what I mean, we are going to share best practices and make technology available - that doesn’t mean we won’t ask to get value back. If we have a patented process we’ll ask for a royalty but we are going to make it available, we are not going to keep it for ourselves.”
Robach said the real measure is implementation.
“How good are we in terms of implementing our programs each and every day, how do we drive food safety culture down through our organisations? That is where we differentiate ourselves from our competition, not by our systems or technology as we are going to share that,” he said.
“Whole genome sequencing has given regulators a whole new set of tools to use to identify potential sources of outbreaks. It has also helped us as we can better map our systems and find out where are those critical points in the system where we might have contamination entering our process.
“The more in-line testing we can do the better we are going to be, if we can catch things earlier we can take corrective action and prevent product going into the marketplace.”
