Speaker Dr Diane Benford, vice-chair EFSA Scientific Committee, told guests food safety experts can only learn about what to do in the future by looking at examples in the past.
Limited information
“We have to expect more instances of adulteration and fraud, risk assessment is needed to assess whether they are a risk to consumers or just a quality issue and frequently that has to be done with limited information,” she said.
“Climate change has the potential to alter our exposure to toxins and other chemicals within the environment and we need the data to do the cumulative risk assessment. With the internet, we cannot be assured of safety and quality and innovation in the food industry and other sectors has the potential to introduce new contaminants into the food chain.
“Risk assessment is required for an increasing number of chemical substances and combinations. We cannot produce the toxicological data needed so we have to look for new methods of obtaining data that we can use and adopt new methods of using the data in risk assessment. There really are challenging times ahead.”
With a background in toxicity and use of in vitro techniques as alternatives to animals, Benford is a toxicologist with expertise in risk assessment.
In 2000, she joined the Food Standards Agency, where she was head of the Risk Assessment Unit until retiring in 2017. The unit has overall responsibility for advice associated with all types of chemicals in food and of microbial contamination.
“When I talk about future challenges I like to separate them as challenges for the risk managers and challenges for the risk assessors because although we try to separate the two, risk management does need to be underpinned by sound risk assessment,” she said.
“There are also challenges specific to the toxicologists when we talk about food chemical risk assessment. In that first category; challenges for risk managers are financial challenges, impact of climate change, challenges presented by internet sales, innovation in the industry and Brexit.”
In terms of financial issues, Benford said we are still living in times of austerity. This can lead irresponsible or reckless producers to adulterate food or produce food fraudulently, which then has authenticity issues. Some of these may be a safety concern, others are more of a quality concern. But as regulators struggle with financial resources they have less opportunity for testing at import and on the market, which is a challenge for everybody.
Melamine infant formula
One major example of food adulteration was 10 years ago when infant formula was adulterated with melamine in China, to make the product look like it had a greater protein content.
“Melamine has a lot of nitrogen in the molecule and the test for protein content was based on nitrogen. Hundreds of thousands of infants became ill, and some died as a result. Fortunately, it wasn’t too much of a big problem in the EU because infant formula was not permitted to be imported from China but there were various food products made with powdered milk that could be imported,” added Benford.
“Fortunately, we haven’t had another example of melamine to this extent since then, but it could happen, and the problem for the risk assessors is having to make decisions based on very limited data.
“In the UK, we had an example of somebody selling what she called edible glitter, to be used in cake decorating (Margaret Martin of EdAble Art, Durham). She made this from shredded plastic and powdered brass and insisted it was safe for consumption. The shredded plastic was polyethylene terephthalate, which yes can be used as a food contact material but that is when it is in an intact form and the surface area in contact with the food is relatively small.

“Once you shred it you cannot be sure that you can extrapolate the risk assessment from one thing to the other. This led to a prosecution in the UK.”
Benford said there is also adulteration in terms of plant products not being what they say they are. She pointed to the example of oregano contaminated with mrytle and olive leaves. Such activity can introduce allergens or pesticides used in the cultivation of non-food plants that are not permitted for food plants to the food chain.
Another case highlighted by Benford was fake rice made with plastic, to make an increased profit from selling food.
The plastic rice, reportedly made from potatoes, sweet potatoes, with synthetic resin moulded into the shape of real rice, allegedly made its way into countries with large rural populations such as India, Indonesia and Vietnam in 2015.
Horsemeat scandal
She said the Horsemeat scandal affected consumers primarily in the UK but was not restricted to the UK. The scare saw horsemeat fraudulently sold as beef. While at the time it was framed largely as a quality issue there was indeed a safety angle with phenylbutazone, which is not permitted in food producing animals, was used in horses that entered the food chain.
“There is still that possibility that something not intended for the food chain does get into the food chain and the risk assessor has to try to do a risk assessment based on limited data. Very often if something is not permitted in food producing animals it’s because the data is not available to ensure the safety in humans,” added Benford.
“With respect to phenylbutazone that was not actually the case because we knew it causes aplastic anemia in humans in a way that you can’t identify at dosage point. EFSA has tried to address this in terms of its opinion on reference points for action on pharmacologically substances that are not permitted in the food chain.
“You can envisage similar things would happen with plant protection products that are not intended for use in the food chain and again the chances are the risk assessor would have limited information available for doing the risk assessment.”
Benford asked what is next in terms of EFSA Reference Points for Action and how can the food safety industry target the limited available resources for monitoring.
“And then, there are the possible impacts of climate change,” she said. “In the UK we have had a strange year, the hottest ever day in March, then it went back to being non-descript, then the hottest ever day in July followed by a non-descript August then the hottest day on August bank holiday (August 26) in between extreme periods of heavy rainfall.
“Those changes in climate can have impacts in very different ways. So for mycotoxins, they are predominantly storage moulds but they can grow in the fields, if the extremes in climate are changing there is a possibility of increased dietary exposure."
Regulated mycotoxins
Deoxynivalenol (DON)
Zearalenone
Fumonisins
Ochratoxin A
Citrinin
Patulin
T2/HT2
Ergot alkaloids
Non-regulated mycotoxins
Nivalenol
Co-occurs with DON, regulation so far not needed
Sterigmatocystin
Phomopsins
Alternariol
Beauvericin
Enniatins
Moniliformin
Diacetoxyscirpenol
“We know that some aflatoxins are genotoxic carcinogens if it is in our dietary exposure it is smaller than we would like and we see frequent non-compliant commodities at import into the EU. We have also seen some aflatoxins in EU-produced products especially in the south of Italy, is this an effect of climate change?”
According to Benford, there are other regulated mycotoxins, ‘a lot of them growing on grains, they grow on crops in the EU not just on crops imported into the EU, and again there is increasing non-compliance’.
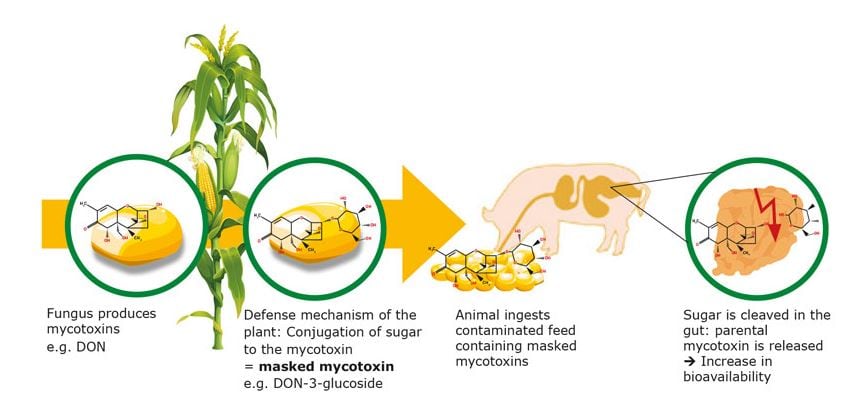
In regard to non-regulated mycotoxins, EFSA has produced scientific opinions on these. In many cases the data is insufficient for risk assessment.
“We need new ways of producing data that is useful for the toxicologists trying to do risk assessment on these substances,” she said.
“There is then the additional challenge of multiple related mycotoxins, that can occur in the same crops. And there is a lack of toxicity data for some of those toxins within a group. And lack of data for the relative potency for some of them. So will exposure increase with climate change? Are all the relevant toxins included in all the regulations? How are we going to do the risk assessments that provide a sound basis for regulation in the future.”
Marine biotoxins
Another example is marine biotoxins Benford said.
“Ciguatoxin was previously seen in non-EU regions in third world countries but has more recently been detected in the EU. Similarly, with tetrodotroxin, which we associate with Pufferfish in Japan. Pufferfish are not permitted for import into the EU but if tetrodotroxin is produced in shellfish or fish harvested off EU waters then that is a different issue,” added Benford.
“Many marine biotoxins are identified on outbreaks of human illness but we don’t have good exposure data because sometimes the toxins are detected some time after the original outbreak of the human illness.
“We need new methods of identifying the toxicity that don’t require large amounts of pure toxin and will not take years to identify and vast amounts of money.
“The possible changes (in marine biotoxins) could be in relation to climate change. The difference in warming of the seas at different times of the year. They could be due to human activity such as shipping. If cargo ships have to travel long distances without a cargo they will ship onboard sea water which is then dumped at their destination which can result in changes in biotoxins. Also the changes in evolving species over time.”
Changes in agricultural practice could also result from climate change, changes in crop production, plant production product usage and exposure, changes in diseases in livestock and contamination by toxin containing weed species, pyrollizidine alkaloids, for example.
Ninety-five percent of PAs are found in five plant families: Asteraceae (Compositae), Boraginaceae, Fabaceae (Leguminosae), Orchidaceae and Apocynaceae.
“These are produced by plants and there are approximately 6,000 plant species worldwide. Over 600 pyrollizidine alkaloids have been identified particularly in honey,” said Benford.
“What’s in the honey depends on where the bees are harvesting the honey. We see pollen dietary supplements marketed for health giving benefits and salad crops like rocket, containing plants. Herbal products marketed for health giving properties, stress, alleviation in dietary supplements and teas that may be contaminated with pyrollizidine alkaloids contaminated plants. The challenges here are out of the hundreds that are identified relatively few have been measured in food. Our exposure estimates are incomplete.”
Speaking about internet sales, Benford said it is very difficult to ensure the quality and the safety of products being sold online.
“Very often they are promoted as alternative therapies, and there are various conspiracy theories that dismiss the expert advice favouring the information that can be found on the internet, for example dinitrophenol (DNP) used as an illegal weight loss substance resulting in the tragic death of some people,” she added.

Bitter apricot kernels marketed as a prevention and cure of cancer contain substances that produce cyanide. Getting round the idea that the experts can’t be trusted is a very difficult issue for us.
Benford said there is a lot of excitement over current technological advances in food production but food safety must not be overlooked, instead, we need new approaches to risk assessment.
“The innovation in the food industry includes nanomaterials, edible packaging and 3D printed products, but very often it is difficult to get information on these that would support risk assessment and some of these are not used directly in food but there is a potential for the transfer of contaminants to the environment, and subsequent uptake into the food chain,” she added.
One example is brominated flame retardants (BFRs). In 2012, EFSA said there were 17 ‘emerging’ BFRs that had been identified in wildlife, food or humans and 10 ‘novel’ BFRs – known applications but not identified in the environment.
“There was potential for some of these to be bioaccumulative in the environment which means it could get into our food. As particular products fade out, others are introduced, so that means tracking exposure is difficult and now we are hearing about mixed halogenated flame retardents,” said Benford.
“We are always playing a game of catch up to see what the industry is doing. BFRs are not added to food but if they are persistent in the environment they will get into food.
“Increasing detection of multiple chemicals in food and the human body worries the consumer. There is insufficient toxicity data on many of those chemicals and there is a limited amount of material for testing certain substances. There are insufficient resources for testing in conventional methods so we need new approaches, we need data to support cumulative risk assessment and we need risk ranking methodology to prioritise areas for testing.
“When will we have enough information to be confident in their use in risk assessment, the link between internal and external exposure is key and to understand whether the effects are adverse or adaptive.”
In conclusion, Benford said EFSA is now starting a new piece of work to try and have a scientific approach to establishing chemical assessment groups.
The Scientific Committee of EFSA published in March 2019 a “guidance on harmonised methodologies for human health, animal health and ecological risk assessment of combined exposure to multiple chemicals”.
EFSA requested the Scientific Committee to develop a scientific opinion addressing scientific criteria for the grouping of chemicals into assessment groups for human risk assessment of combined exposure to multiple chemicals, taking into account:
- The scientific principles laid down in the recent scientific committee guidance on “harmonised methodologies for human health, animal health and ecological risk assessment of combined exposure to multiple chemicals” as well as other relevant cross-cutting guidance documents (weight of evidence, biological relevance, uncertainty).
- The context of the risk assessment (prioritisation, urgent risk assessment, pre- and post-market risk assessment), data availability, time and resources for the grouping of chemicals defined in the problem formulation.
- Tiering principles and a range of fit for purpose scenarios should be developed, considering available hazard information (e.g. reference points, specific toxicological effects in target organs, mode of action) and exposure information. Additional considerations may be of relevance including adverse outcome pathways, toxicokinetics and human biomonitoring.
- Relevant EFSA sectoral regulatory provisions and activities including the work on cumulative assessment groups (CAG) for pesticides by the Pesticide units, relevant risk assessment activities on contaminants, any other relevant panel (FEEDAP, FAF, CEP, NDA) and other related European activities (European Commission, JRC, ECHA, EMA, EDC-MixRisk and EUROMIX Horizon 2020 projects).
- Relevant international activities including the recent guidance documents of the OECD and the practical approach developed during the WHO/FAO consultation s to be piloted by JMPR and JECFA in 2019. This will ensure consistency and harmonisation, provide an international dimension to the statement and avoid duplication of the work.
In line with EFSA’s policy on openness and transparency (EFSA Strategy 2020), EFSA will publish a draft version of the scientific opinion for public consultation. Following the public consultation, the finalised opinion will be published after adoption by the Scientific Committee together with the technical report of the public consultation, delivered to the Scientific Committee by spring 2021.
“We need approaches to prioritising and predictive modelling to make sure we are doing (chemical) mixtures assessments in the best possible way. And then there are new approaches to the microbiome, we have known for years the microflora in the gut can be responsible for metabolic activation and detoxification of chemicals. What we know now is the microbiome is extremely variable both between individuals and in the same individual at different times. We haven’t yet got a handle on how to use that in risk assessment,” she added.
In terms of ‘big data’ and social media, Benford said artificial intelligence (AI) is a concern.
“With AI we worry about transparency. AI is dependant on who is doing the teaching and who is putting the information into the computer. Can we trust that for food safety assessments? If you have enough data does that mean you overcome the limitations of each individual piece of data.
“In the future, there will be increasing interest in using novel methods of collecting data, but risk assessors need to be confident that the data is robust.”




