BioMérieux reveals culture media compliance progress
The firm has looked at requirements of the updated version and the majority of media intended for foodborne and the water analyses are already manufactured and tested with the requirements. Those that were not have been or are being brought into compliance.
The standard was published at ISO level first edition in May last year and the corrected version in November but EN ISO official publication was in January 2015.
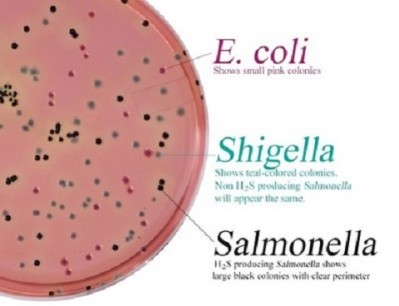
Quality control of media
Isabelle Desforges, bioMérieux scientific manager of food, said labs and manufacturers usually have six months to apply it.
“There is a need to check and compare, for each media, the current quality control (QC) applied (if compliant with the previous version of ISO 11133 standard or not) with the request of the new standard.
“It is also necessary to check on several production batches that the media give conform results when the new QC (if different than the current) is applied.
“The main differences between previous and the new version can be the number and reference of QC strains to test, inoculum concentration level, criteria of acceptation but also the reference media to use for productivity test.
“Some media (new or missing) were not in the previous version and have to be compliant with the new standard.”
Read more about the standard in a guest article from Barbara Gerten, an application training scientist at Merck Millipore, who was involved in the standards revision.
The aim is to guarantee labs have a high reliability of analyses and allow them to justify simplification of performance controls by avoiding systematic checks of each batch of ready to use media at reception.
EN ISO 11133:2014 standard is a full, self-contained standard, which includes culture media dedicated to the analysis of food and water and is recognized by accreditation bodies.
EN ISO is mandatory to apply in Europe but implementation is at the discretion of local organizations of accreditations that can prescribe slightly different rules.
It targets professional culture media manufacturers but is also directed towards labs producing their own culture media in-house.
Compliant media
Desforges, a member of AFNOR, ISO and CEN committees, said bioMérieux started the implementation process media per media and will finish it by next month.
She is also part of the ISO/TC34/SC9/WG5, the working group in charge of the revision of ISO 11133.
“Today, the great majority of media QC are in compliance (new QC procedure validated, first compliant lots released and new certificates available).
“It is not an experimental standard anymore but a full standard which is well evaluated, it covers media for food analysis but also for water analysis, it’s a full one part document with several annexes for details and explanations.”
Desforges said the standard was looked at by an independent body, with the help of manufacturers, to set minimum requirements for all labs preparing their media by themselves.
“If a manufacturer is able to prove its media are compliant, labs users of such media will have a guarantee of media quality then of results of analyses and simplification of QC at reception.
“In addition, if a manufacturer is able to provide assistance for the implementation of the standard by a lab, it can strengthen the relationship of trust.”