For routine indicator organism testing, most labs rely on traditional media and methods. There are now several types of convenient media on the market today claiming to rival the classical methods:
- How do these alternative methods improve your microbial testing workflow?
- Are they just as reliable as traditional methods, particularly with difficult matrices?
What are alternative indicator organism testing methods?
Alternative methods are used for rapid routine testing of microbial contamination in food and beverage samples throughout production, from raw materials, to finished products. They tend to be based on chromogenic media and can give faster results while reducing the need for time-consuming agar media preparation time.
These convenient media solutions hold many advantages over traditional methods, including:
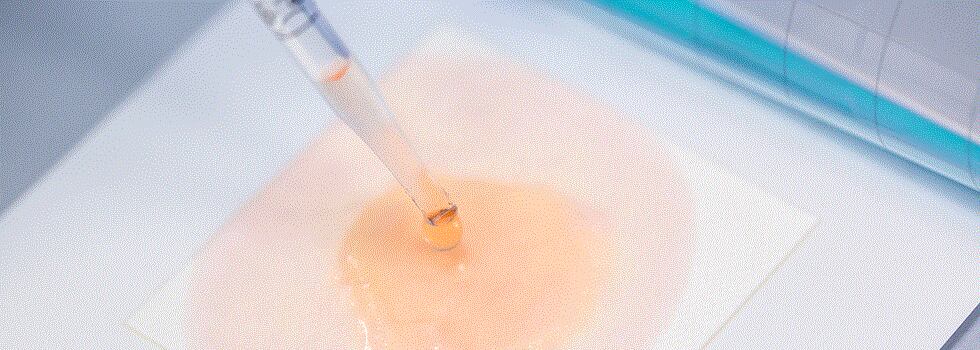
Automatic sample diffusion: simply pipet 1 ml to the center of the pad, it will diffuse automatically, without the need for costly single use spreaders, or flaming of reusable spreaders.
Faster time to results: particularly with yeast and mold contamination, these microorganisms tend to grow slowly, however, with these convenient methods, results can be obtained in 48 hours instead of ~72 hours.
Reduced risk of contamination: lids on most convenient media solutions help prevent cross-contamination.
Long shelf life: improve your inventory management with a shelf-life of up to 36 months in comparison to four to six months for traditional culture media plates.
Storage and incubation: some convenient media types don’t require cold storage, and due to their small size, they take up less space, both in stock and in the incubator.
Reduced waste: decrease biohazard and plastic waste and disposal of out-of-date plates.
Compliant: most convenient methods are validated according to international standards. For example, the MC-Media Pad from Merck KGaA, Darmstadt, Germany, all received PTM or OMA certification from the AOAC Research Institute as a Performance Tested Method.
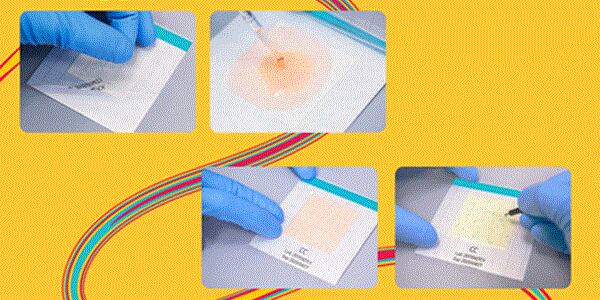
Workflow example with MC-Media Pad: just inoculate sample to the pad, close the lid, incubate and count.
How well do they perform when testing difficult matrices?
Certain samples can be tricky to work with, these include samples with enzymatic activity, high fat, salt or sugar content, high viscosity or acidity or strong coloration.
Occasionally, it is necessary to carry out an additional 1:10 dilution step in the protocol in order to ensure that the colony-forming unit is low enough to count, but also to remove background coloration to improve colony contrast easing enumeration.
In general, convenient media can be used for a vast range of food and beverage samples, including difficult to test matrices. Take a look at our application note where we tested the MC-Media Pad system to see how well they’d perform.
Conclusions
- Traditional media methods have been the standard in most labs for many years, but do not meet all lab needs
- Ready-to-use methods can streamline the lab workflow to offer better time and lab budget management
- Rapid microbiological methods provide many benefits: faster time to result, easy handling, long shelf life, reduced storage space
- Many of these convenient solutions are compliant to international standards
Download the application note for full details of the results.