Fermentation may be a millennia-old technique, but it continues to throw up new opportunities in food production. From providing new protein sources or health-boosting ingredients to improving the taste, texture and safety of food products, fermentation adds value across the food industry.
René Floris, Food Research Division Manager at NIZO and member of the FoodNavigator Advisory Panel, asks Janneke Ouwerkerk, microbiome and fermentation expert at NIZO, to explain how.
René Floris: Why is food fermentation such a hot topic in the food industry right now?
Janneke Ouwerkerk: Food fermentation refers to any process in which the activity of microbes causes a desirable change in a food. People have used fermentation in food production since Neolithic times, creating a wide range traditional foodstuffs from beer and alcoholic drinks to a variety of pickles, kombucha and yoghurt. Recently, trends such as the protein transition and ‘clean label’ products have encouraged the food industry to take a fresh look at this ancient technique and explore many new applications.
RF: What kind of new applications are being explored?
JO: Traditionally, fermentation has been used to create beneficial changes in food, for example to create alcohol, help bread rise or preserve food. And this kind of application is still common in the world of the protein transition, healthy eating and clean label products. For example, fermentation is often used to add a pleasing depth of flavour to low-fat cheeses and plant-based cheese alternatives. Moreover, our own research at NIZO suggests that fermentation can remove the volatile organic compounds that cause the off-flavours often associated with plant-based protein ingredients, such as the hexanal responsible for the ‘beaniness’ of legume-based proteins. We have also used fermentation to successfully acidify plant-based cream cheeses, providing better protection against mould growth than standard chemical acidification. In all these cases, fermentation allows a desired result to be achieved using natural processing, without resorting to chemical additives.
But fermentation is also increasingly being used to directly manufacture food ingredients and components. Perhaps the most obvious application here is using fermentation to grow a biomass of microbes that act as the food stuff themselves, either as a probiotic to promote health benefits for the consumer or as an alternative source of protein such as Quorn. Even more recently, we have seen interest in so-called ‘precision fermentation’ where microorganisms are modified to produce a protein with an identical amino acid sequence as a target animal protein such as caseins and egg albumin.
RF: Do these new applications also use new fermentation approaches?
JO: Very much so. For example, plant-based ingredients and products contain different sugars and fats as well as different proteins from dairy so you can’t simply translate familiar fermentation processes. Meanwhile, when creating microbial proteins, the goal is to grow biomass as fast as possible which is a very different focus to previous fermentation approaches.
In fact, many of these new applications are based on “new” microbes. For probiotics, that often means identifying novel microbial species or strains that have specific desired traits and don’t have unwanted traits like antibiotic-resistance. Here, in silico screening is a valuable tool for quickly narrowing down the multitude of candidates. In contrast, precision fermentation involves microbes creating proteins they wouldn’t naturally make. This currently means that proteins from precision fermentation can only be marketed in certain territories, such as the European Union, if food product risk assessment has been properly performed.
As I mentioned, plant-based products offer a very different environment to animal-based ones. So here too it is often necessary to adapt known cultures to suit this new environment. To avoid the market entry issues around GMOs, it is possible instead to use accelerated evolution. This involves identifying a promising strain, then repeated inoculation on the desired substrate, potentially guided by genome sequencing to monitor changes in metabolic capabilities, to create a new strain that is better adapted to the (plant-based) environment.
RF: What are the challenges of working with novel microbes?
JO: There are many challenges on the road from the right micro-organism to a commercial fermentation process. And it is important to consider them early in the development journey to reduce the risk of longer delays further down the line.
One of the key ones is manufacturability: Can you grow your chosen microbe fast enough to support volume manufacturing with high stability and ensuring food grade production? In fact, you should start to address this already in the discovery phase when you are identifying the right microbe. High throughput screening allows you to rule out microbes that grow too slowly or die off too quickly, while in silico genome analysis can highlight potential food safety issues such as genes associated with antimicrobial resistance, toxin production or pathogenicity
When using any form of novel micro-organism, it is important to note that, even if there are no viable organisms in the end application, the DNA can remain present. Thus, there is a possibility of novel genes transferring to micro-organisms in the environment and precautions must be taken. This could involve using computer modelling and bioinformatics to assess the risks posed by novel genes or even reverting to using (less-optimised) organisms from regulators’ approved lists of safe microbes such as the EFSA’s qualified presumption of safety (QPS) list.
RF: Once you have identified the right microorganism, what next?
JO: The next step is to design a fermentation process based on your microbe that will work effectively on a commercial scale. This has to be done in a controlled, stepwise progression: from lab-scale through small prototyping and pilot production before final transfer to volume production. Remember that microbes are living organisms that respond to their environment, so it is important to understand how each step in this progression impacts the growing conditions for microbes during fermentation.
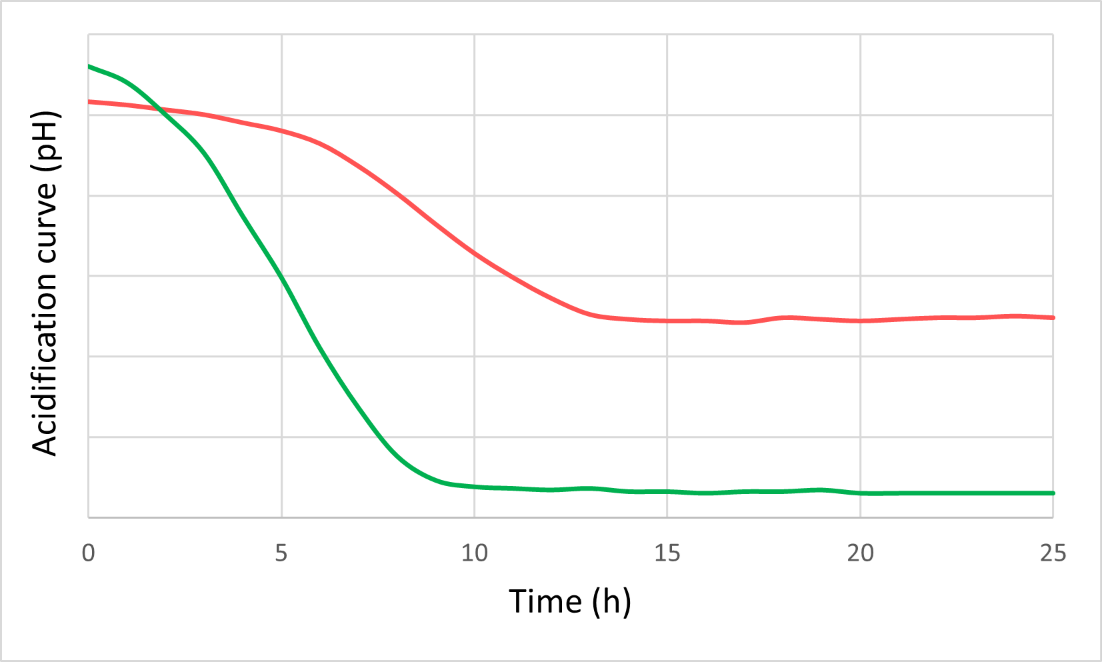
To maximise growth rates, the culturing medium and conditions should be optimised which requires investigation of the interaction effects of various medium components, pHs and cryoprotectants. Data science helps provide insight from in vitro experiments, distilling data from different equipment and assays into clear guidance on the best culturing conditions.
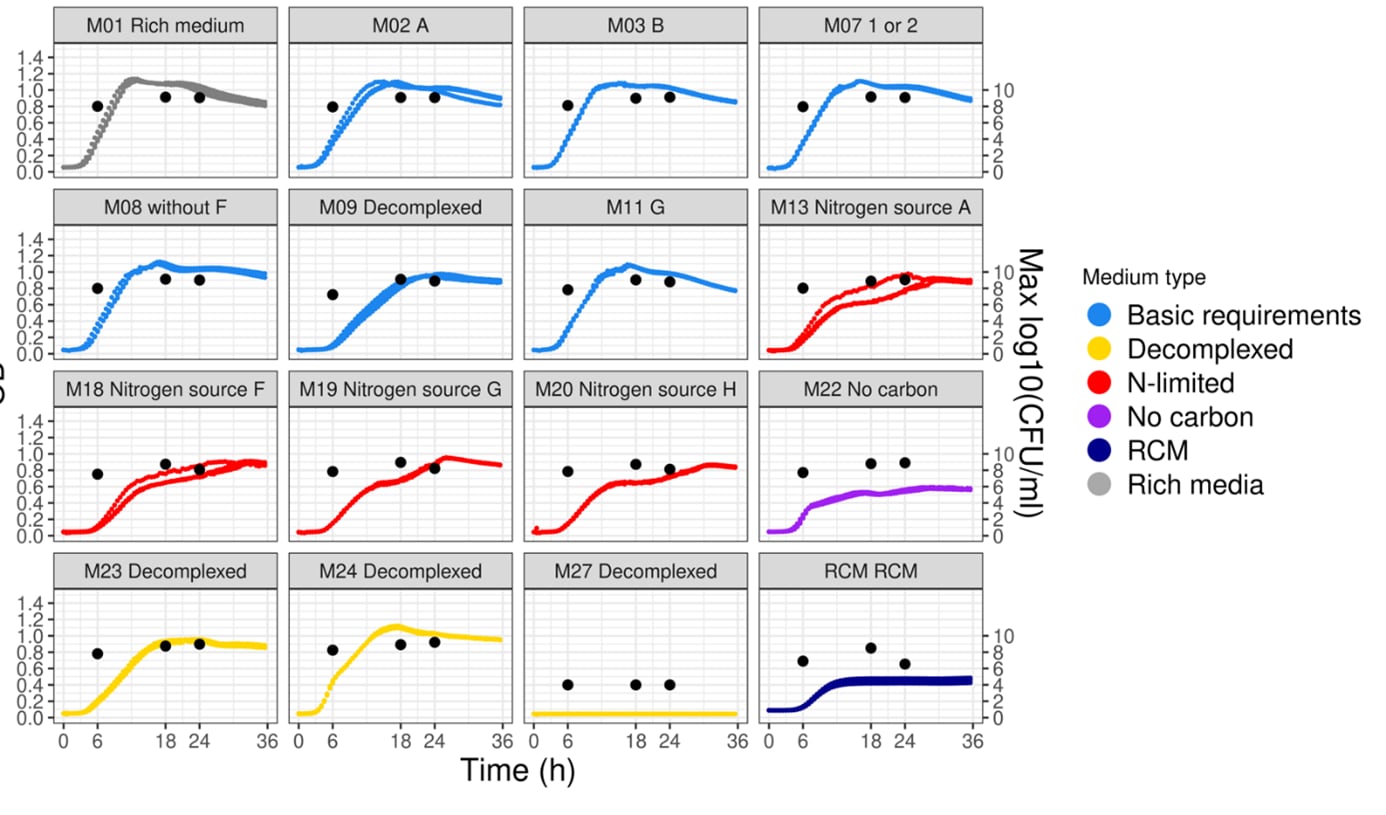
This medium optimisation is usually carried out at the lab scale where fermentation volumes are at the order of magnitude of microlitres to litres. Production is then scaled-up to tens and then hundreds of litres to explore downstream options (such as centrifugation and cross-flow filtration). Once all these conditions have been optimised, fermentation can move into the pilot phase with fermentation volumes in the thousands of litres, allowing formulations for food-grade products. This step-by-step upscaling is critical, because even small changes can have a big impact on how the microbe reacts.



