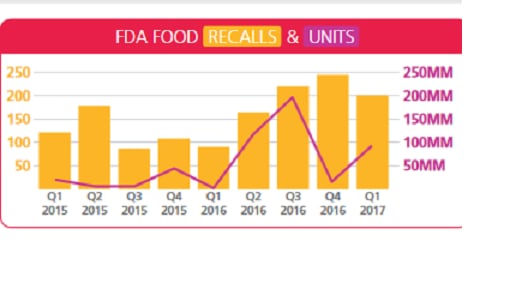
FDA food-related recalls dropped 19% in Q1 to 200, but the number of actual units increased 507% to about 92 million products.
The spike was mainly driven by nutritional supplements, which accounted for more than 80% of food units recalled, mostly due to one large recall.
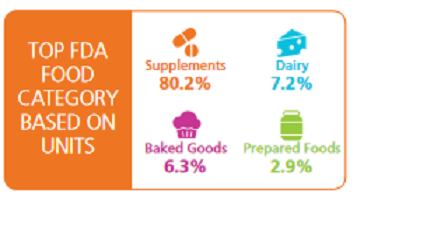
Kingsway Trading Inc. recalled “Xanthium & Siler Combo (Bi Yan Pian) Dietary Supplement” because it contained banned Ephedra Alkaloids in February and A&H Focal Inc. took action on 21 products which could contain undeclared erectile dysfunction ingredients in the quarter.
FDA product categories based on recalls were prepared foods (16%), baked goods (14.5%) and dairy (10.5%).
Supplements (28.2%) and baked goods (27.9%) combined to make up 56.1% of FDA recalled units in Q4 2016.
Recalls down, units up
Michael Good, VP of marketing and sales operations, said Q1 was a ‘mixed bag’ for the food and beverage industry.
“On the one hand, recalls dropped 19%, and it’s the first time we’ve observed a decline in that measurement since Q1 2016,” he told FoodQualityNews.
“On the other hand, the number of units recalled increased. And even when you isolate the large supplement issue, recalled units still would have gone up compared to Q4 2016.”
The 507% increase wasn’t entirely related to supplements but was a substantial portion of it, said Good.
“While this category doesn’t often lead, in 2016 supplements were the fifth highest category in recalls and fourth highest in terms of recalled units. If companies can improve their supply chain track and trace methods, that can help them isolate the issue and reduce the impact of the recall.
“In Q1 2017 there were five recalls with more than one million units each. In Q1 2016 there were none. In fact, four of those five recalls each accounted for more units than all the recalls in Q1 2016 combined.”
Reasons behind the recalls
Top FDA food recall causes based on units were quality issue (80.1%), bacterial contamination (11.6%), undeclared allergen (7.2%) and other (1.1%).
As well as the supplement recall, there were other quality issues in Q1, including premature spoilage, uneviscerated fish, packaging defects, undercooked chicken, and an “off” odour and taste.
The two main causes of bacterial contamination recalls were Listeria and Salmonella. Salmonella affected more recalls (64.9% vs. 32.5%) but Listeria was behind more recalled units (81.9% vs. 18%).
“Bacterial contamination made up a lower percentage of recalled units because the supplement issue dominated,” said Good.
“In fact, if you remove that recall, bacterial contamination would have accounted for nearly 54% of recalled units.
“Looking at the number of recalls, bacterial contamination was the leading cause with 38.5% of FDA food recalls. By contrast, quality issues accounted for just 12% of FDA recalls.”
The number of international FDA food recalls shifted from 23 (9%) in the last quarter to 21 (11%).
USDA recalls ‘consistent’
Poultry was the top USDA recall category for the second quarter in a row and foreign materials and misbranding accounted for more than 82% of recalled pounds.
USDA recalls stayed at 32, while recalled pounds declined 10% to 2.5 million.
“USDA recall activity stayed fairly consistent from Q4 2016 to Q1 2017, with recalls staying the same and recalled pounds dropping just 10%,” said Good.
“For the second quarter in a row, the main category was poultry, which accounted for 80.8% of the recalled pounds. The top causes based on recalled pounds were foreign materials at 41.8% and misbranding at 40.5%.”
