EFSA approves safety of GM oilseed rape

The herbicide-tolerant oilseed rape has been developed by Bayer CropBioscience to be resistant to glufosinate-ammonium-containing herbicides. The EFSA Panel on Genetically Modified Organisms was tasked with assessing safety of foods and feed containing or consisting of the crop, with the exception of processed oil. European cultivation of the crop was not included in the assessment process.
The Panel said in the EFSA Journal that the GM seeds are “unlikely to have an adverse effect on human and animal health or on the environment, in the context of their intended uses.”
It said that the import of oilseed rape Ms8, Rf3 and Ms8 x Rf3 for feed use, even allowing for a worst case scenario in which the seed might be spilled, would not pose “a significant and imminent risk to the environment.” In addition, it found in a study with broiler chickens that the seed was just as nutritious as non-GM oilseed rape.
From a food safety perspective, the Panel assessed the composition of the crop in comparison to a non-GM variety and, other than the new proteins, found no significant difference between the two.
“The safety of the new proteins and the whole food was evaluated with respect to potential toxicity, allergenicity and nutritional wholesomeness,” it said, and found “no indication of potential concerns”.
EFSA has already assessed the safety of the newly expressed proteins present in oilseed rape Ms8, Rf3 and Ms8 x Rf3 in 2005 and 2009, and had not identified any safety concerns for humans or animals in the context of intended usage.
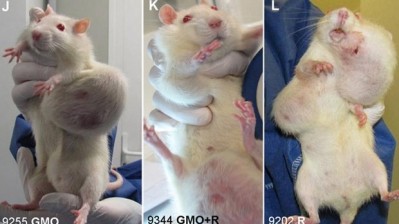
The approval comes just days after a study of Monsanto’s glyphosate-tolerant NK603 maize suggested rats fed the maize may develop more tumours than rats fed a diet without the GM maize. The research has proved controversial, and the European Commission has asked EFSA to review the study. EFSA has said it will issue a preliminary review next week.